
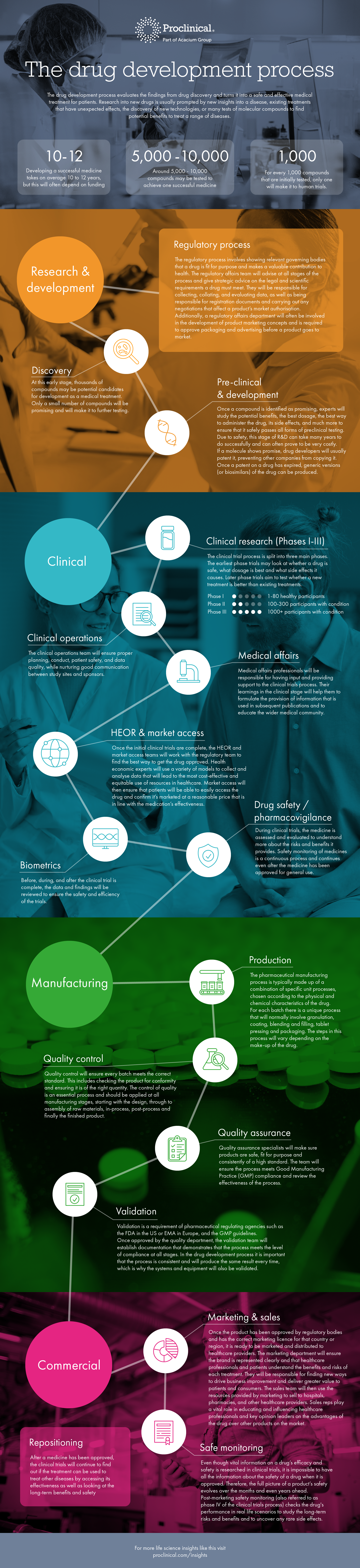
The drug development process turns a compound that is identified in drug discovery and turns it into a safe and effective medicine.
The journey to creating a new treatment begins with research and development. Research into new drugs is usually prompted by new insights into a disease, existing treatments that have unexpected effects, the discovery of new technologies, or many tests of molecular compounds to find potential benefits to treat a range of diseases.
Once a compound is identified as promising and safely passes all forms of preclinical testing, clinical researchers will then look at whether a drug is effective, what dosage is best and what side effects it causes. Once the initial clinical trials are complete, the HEOR and market access teams will work with the regulatory team to find the best way to get the drug approved.
When the drug has safely passed its way through the clinical process, it will then be manufactured. The pharmaceutical manufacturing process is typically made up of a combination of specific unit processes, chosen according to the physical and chemical characteristics of the drug. This process will vary but is typically made up of a combination of specific unit processes, chosen according to the physical and chemical characteristics of the drug. Quality control and assurance specialists will make sure products are safe, fit for purpose and consistently of a high standard. Once approved by the quality department, the validation team will establish documentation that demonstrates that the process meets the level of compliance at all stages.
Once the product has been approved by regulatory bodies and has the correct marketing licence for that country or region, it is ready to be marketed and distributed to healthcare providers.
Even though vital information on a drug’s efficacy and safety is researched in clinical trials, it is impossible to have all the information about the safety of a drug when it is approved. Post-marketing safety monitoring, also referred to as phase IV of the clinical trials process, continues to monitor how the drug performs and studies any rare side effects.
See our infographic to discover more on what it takes to get a medicine brought to market:
.png)





.png)

.png)
.png)

.png)
.png)













